The Repackaging Scam: How Imported Medical Devices Enter the US Market as 'Genuine'

The surgical hernia mesh looked perfect. It bore the branding of a trusted manufacturer, arrived in pristine packaging, and landed at a major US hospital ready for implantation. Yet this device, imported and sold by RAM Medical, Inc., was a counterfeit, contaminated with microorganisms that could turn a routine procedure into a life-threatening infection. This was a window into a sophisticated shadow supply chain where fraudulent actors exploit regulatory gaps to flood American hospitals with counterfeit, misbranded, and potentially lethal medical devices.
Understanding the Grey Market of Medical Devices in the US
At the heart of this crisis lies geographic arbitrage, the engine driving an underground economy in medical devices. Medical device manufacturers often sell identical products at vastly different prices across global markets. A cardiac monitor costing £8,000 in the United States might retail for £3,500 in Southeast Asia. Fraudulent operators exploit this by purchasing authentic devices in lower-cost markets, illegally importing them into the US, and reselling them at American prices while pocketing the difference.
One of the major examples of this is the case of Advanced Inventory Management, Inc. (AIM). Employees used ordinary household hairdryers to heat and peel away labels from imported medical products stating "not for resale in the United States." This low-tech method stripped away critical warnings that would have flagged these devices as grey market imports.
Once removed, AIM resold the devices to unsuspecting US customers at profit margins between 35% and 50%. The scheme was staggeringly simple, requiring no sophisticated technology. Federal investigators discovered AIM had realised approximately £400,000 in illegal profits whilst introducing countless unapproved devices into sterile operating theatres.
How Counterfeit Medical Devices Enter the US Market
International Mail and Express Couriers: Small shipments arrive daily through postal services, often mislabelled as personal items to avoid customs scrutiny. During COVID-19, this channel exploded as demand for PPE overwhelmed traditional supply chains.
Port Manipulation: Fraudulent importers deliberately route shipments through different ports, exploiting variations in inspection resources. A container rejected at Los Angeles might be rerouted to Miami, where inspectors may be less familiar with specific products.
Legitimate-Looking Distributors: Counterfeiters establish distribution companies with professional websites and sales staff. During the pandemic, Group Purchasing Organisations (GPOs) vetting suppliers for America's 7,000-plus hospitals reported that 60% of member hospitals received grey market solicitations. When one GPO reviewed over 2,500 unique grey market suppliers, only 1% were viable.
Corporate Complicity: The threat escalates when deception originates from manufacturers themselves. A whistleblower lawsuit against Biotronik alleged the company concealed battery defects in BioMonitor 3M heart-monitoring devices, selling them in the US with 18-month warranties whilst offering three-year warranties globally, knowing batteries failed around 20 months. In 2022, Biotronik paid nearly £10 million to settle similar claims.
The Legal Framework: Misbranding and Adulteration
The Federal Food, Drug, and Cosmetic Act defines two critical violations.
The FDA distinguishes between "repackagers," who change physical packaging, and "relabelers," who modify existing labelling. Relabelers are classified as manufacturers, subjecting them to full federal oversight: Good Manufacturing Practices compliance, FDA facility registration, and device database listing. This regulatory firewall is precisely what illicit actors bypass.
The Scale of Imports: A Massive Vulnerability
Approximately 40-50% of medical devices sold in the US are manufactured abroad, with significant volumes from China, Mexico, Malaysia, Ireland, and Germany. This complex, multinational supply chain creates numerous fraud opportunities. A legitimate device manufactured in Germany might be diverted to a grey market operator in Eastern Europe, shipped to a repackaging facility in South America, and then enter the US through a port with limited inspection capacity.
The 510(k) Process and Its Exploitation
Medical devices entering the US market navigate the FDA's 510(k) pathway, requiring manufacturers to demonstrate their device is "substantially equivalent" to a legally marketed predicate device. Approximately 3,000 applications are submitted annually, with clearance taking three to twelve months.
Because 510(k) clearance is device-specific and company-specific, a legitimate device approved for one manufacturer cannot legally be sold by another entity without separate clearance. Grey market importers deliberately obscure this by removing or altering labelling, introducing products that may be genuine in composition but illegal in distribution.
FDA Border Detection and the Safeguarding Therapeutics Act
The FDA employs risk-based algorithms to flag shipments based on country of origin, shipper history, and fraud patterns. Investigators look for repackaging telltale signs: inconsistent labelling, adhesive residue, heat application evidence, and mismatched lot numbers.
However, a critical loophole hampered enforcement for years. Whilst the FDA could seize and destroy counterfeit drugs, regulations required counterfeit medical devices be returned to the sender. Fraudulent importers simply repackaged rejected shipments and sent them back through different ports.
The Safeguarding Therapeutics Act of 2020 closed this gap, giving the FDA authority to seize and destroy counterfeit medical devices at US borders, permanently removing dangerous products from the global supply chain.
How to Check If Medical Devices Are Genuine
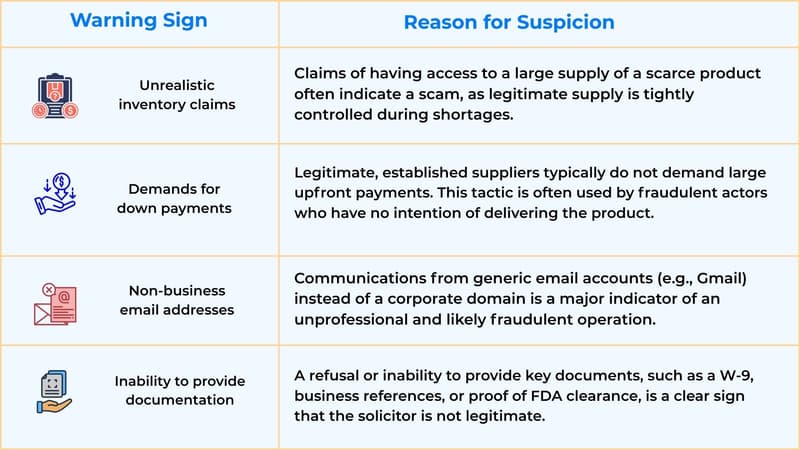
Verify the Unique Device Identifier (UDI): The FDA's UDI system assigns distinct codes to medical devices. The publicly accessible Global Unique Device Identification Database (GUDID) allows procurement officers to verify device identifiers against official records.
Examine Packaging: Authentic devices feature consistent, high-quality printing with no misspellings or blurred text. Packaging should show no tampering signs: no adhesive residue, heat damage, or re-sealing.
Verify Distributor Authorisation: Contact manufacturers directly to confirm distributors are authorised partners. Any reluctance to provide this information indicates fraud.
Demand Documentation: Legitimate suppliers readily provide certificates of conformity, FDA clearance letters, and chain-of-custody documentation.
Brand Protection and Advanced Authentication Technologies
Traditional anti-counterfeiting measures like holograms suffer from a critical vulnerability: sufficiently sophisticated counterfeiters can copy them. This has driven the development of advanced solutions like Origin by Acviss, employing non-cloneable authentication methods that create unique, physically uncopyable identifiers. Unlike printed codes that can be reproduced, non-cloneable technologies leverage physical properties at microscopic or molecular levels that cannot be duplicated.
Product authentication and verification systems enable supply chain management through complete product traceability from manufacture to point of care. Track and trace capabilities allow manufacturers to monitor product movement in real-time, identifying grey market diversions before counterfeit devices reach patients. These systems create immutable records, preventing fraudulent actors from introducing counterfeits into the digital chain of custody.
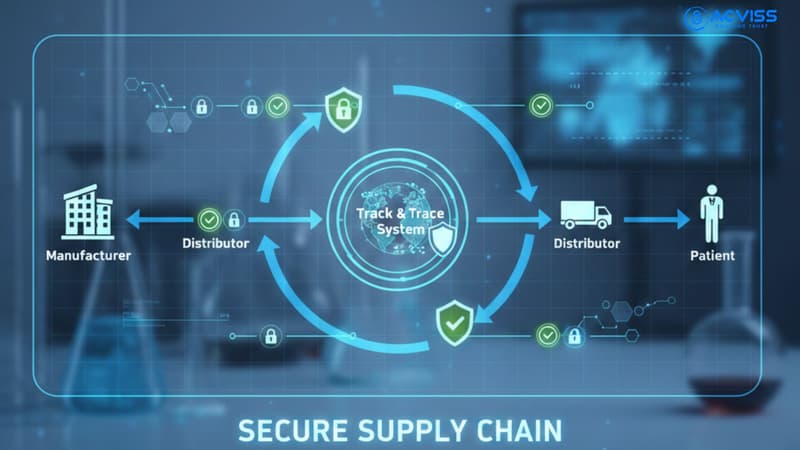
From a customer engagement perspective, authentication technologies empower end users to verify product authenticity through smartphone scans, building trust whilst creating feedback loops alerting manufacturers to counterfeiting attempts in real-time.
The Direction of Regulation and Enforcement
Regulators continue to explore stronger controls on imported counterfeits, including enhanced data-sharing and tighter oversight of foreign suppliers.
However, regulation will always lag behind innovation. The responsibility for product safety increasingly sits with those who control the design of the supply chain, not just those who police it.
Securing Authenticity in a Global Market
Repackaging scams succeed because they exploit complexity, distance, and assumed trust. They turn genuine products into liabilities and expose brands to risks they never authorised.
The solution is not fear-driven compliance. It is structural visibility.
Product authentication, brand verification, and non-cloneable track and trace systems are no longer defensive tools. They are strategic enablers for product safety, regulatory confidence, and long-term brand protection.
If you are interested in learning more about how product traceability, anti-counterfeiting technologies, and verification frameworks can strengthen your supply chain and protect your brand, get in touch with us. The conversation begins with visibility, and it ends with trust that can be proven.
